Empowering Your Path to Successful Clinical Trials

Your Trusted Partner in Clinical Research Success
Foss Medical Consulting is dedicated to supporting clinical research organizations in navigating the complexities of clinical trials with clarity and efficiency. Our mission is to provide organizations with the guidance they need to conduct successful, compliant trials that deliver meaningful results. We understand the critical role these studies play in advancing healthcare, and we’re committed to helping you achieve reliable outcomes that make a real difference.
Our approach is built on a foundation of careful planning, streamlined processes, and a commitment to compliance at every stage. Each clinical trial presents unique challenges, and we tailor our strategies to meet the specific goals of each project. By working closely with your team, we ensure that every step is well-organized, clear, and aligned with the highest standards, allowing you to focus on the research while we handle the complexities.
At Foss Medical Consulting, we pride ourselves on being more than a service provider—we’re a partner in your clinical research journey. With a dedication to precision and quality, we bring a depth of knowledge to every project, guiding you from trial planning to completion. Let us help you transform your vision into impactful research that advances healthcare for all.
Medical Domains in Clinical Research
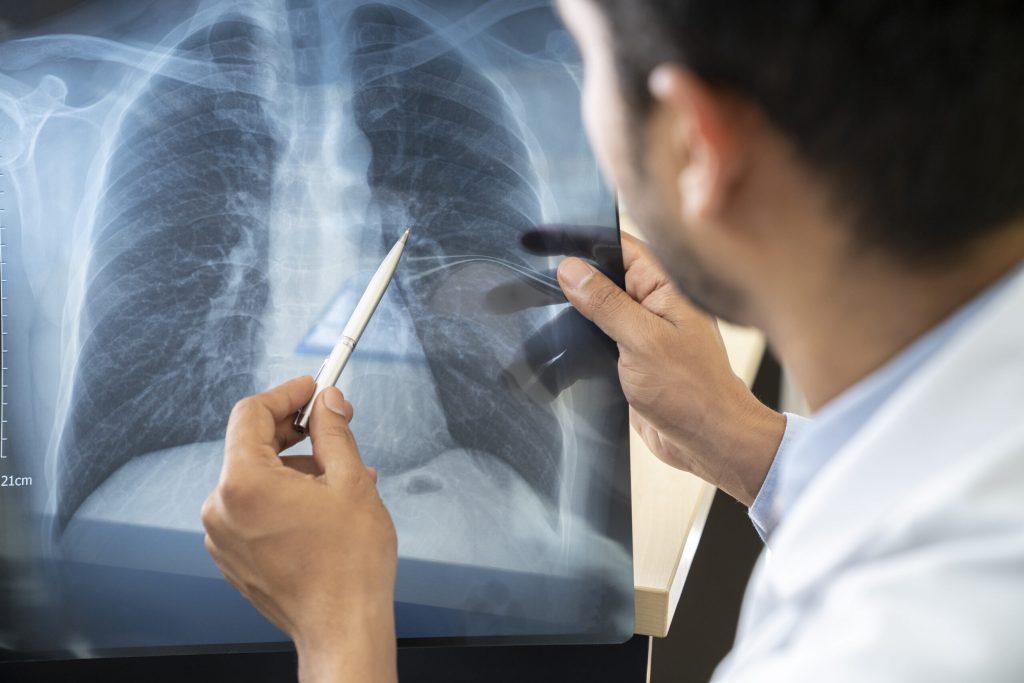
01. Pulmonology
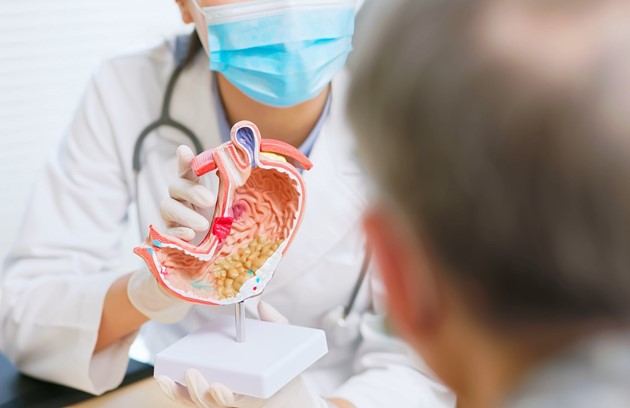
02. Gastroenterology
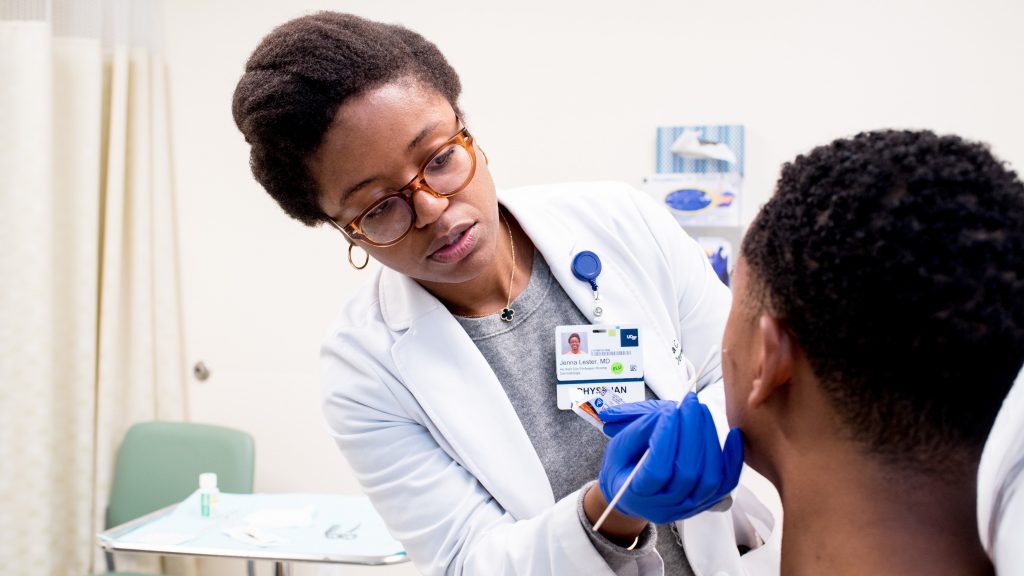
03. Dermatology
04. Rheumatology

05. Nephrology

06. Ophthalmology

Take the Next Step in Your Clinical Trial Journey
Our Proven Process for Clinical Trial Success
01
Initial Consultation
We begin by understanding your specific needs, project goals, and trial requirements to tailor our approach to your unique research.
02
Strategic Planning
We develop a comprehensive plan, outlining the key stages, regulatory guidelines, and timelines to ensure a smooth and efficient trial process.
03
Execution and Monitoring
Our team manages the day-to-day operations, monitoring progress closely to ensure adherence to protocols and addressing any issues promptly.
04
Data Analysis and Reporting
Once the trial concludes, we provide detailed analysis and reports, offering valuable insights to support your next steps and ensure compliance with regulatory standards.
Frequently Asked Questions
Start Your Journey By Talking to Our Experts
- 321-461-8583
- 500 44th St NE Washington, DC 20019
- info@rafaelfoss.com
Why Work With Us?
At Foss Medical Consulting, we stand out for our unwavering commitment to the success of your clinical trials. Our team brings a deep understanding of the clinical research landscape, paired with a passion for supporting clients through every phase of their trial journey. We work closely with you to understand your unique needs, ensuring that each trial is tailored to meet your specific goals and regulatory requirements.
We believe in a collaborative approach, combining your expertise with our comprehensive knowledge to streamline processes and overcome any challenges that may arise. Our focus on clear communication, precision, and compliance ensures that your trial progresses smoothly, with consistent updates and solutions for any obstacles. We are committed to delivering timely, accurate results that drive real-world impact.
Choosing Foss Medical Consulting means partnering with a team that values integrity, transparency, and client success. Our proven track record of helping organizations navigate complex trials speaks to our dedication and reliability. We are here to provide you with the guidance, expertise, and support you need to turn your clinical trial vision into a reality.
Our Core Values
01. Integrity
02. Collaboration
03. Precision
04. Innovation
What Our Clients Say
- Dr. Emily ThompsonFoss Medical Consulting provided invaluable support throughout our clinical trial. Their team is efficient, knowledgeable, and committed to delivering results. We couldn’t have asked for a better partner in our research.
- John MillerThe team at Foss Medical Consulting ensured our trial ran smoothly, from recruitment to data analysis. Their attention to detail and dedication to compliance were crucial to the success of our study.
- Sarah DavisThanks to Foss Medical Consulting, our clinical trial was well-organized and met all regulatory requirements. Their professional guidance helped us navigate complex challenges with ease.
- Dr. Michael RobertsI’ve worked with many trial support teams, but Foss Medical Consulting stands out for their reliability and commitment. They provided timely updates and ensured the trial stayed on track.
- Anna GarciaWorking with Foss Medical Consulting was a game changer for our clinical study. Their team’s ability to handle every phase of the trial process allowed us to focus on our core research objectives.
- David LeeThe level of professionalism and expertise Foss Medical Consulting brought to our clinical trial was outstanding. Their strategic approach helped us navigate a challenging project and achieve our goals
